Would you like to work in a leading global specialist environment that works with the development of cancer medicines of tomorrow?
With us you will have the opportunity to work as part of a unique environment that is involved in the entire value chain, from the preclinical phase to the commercial production and distribution of radiopharmaceuticals.
Vacancies from Agilera webcruiter will appear here:
A forward-looking employer
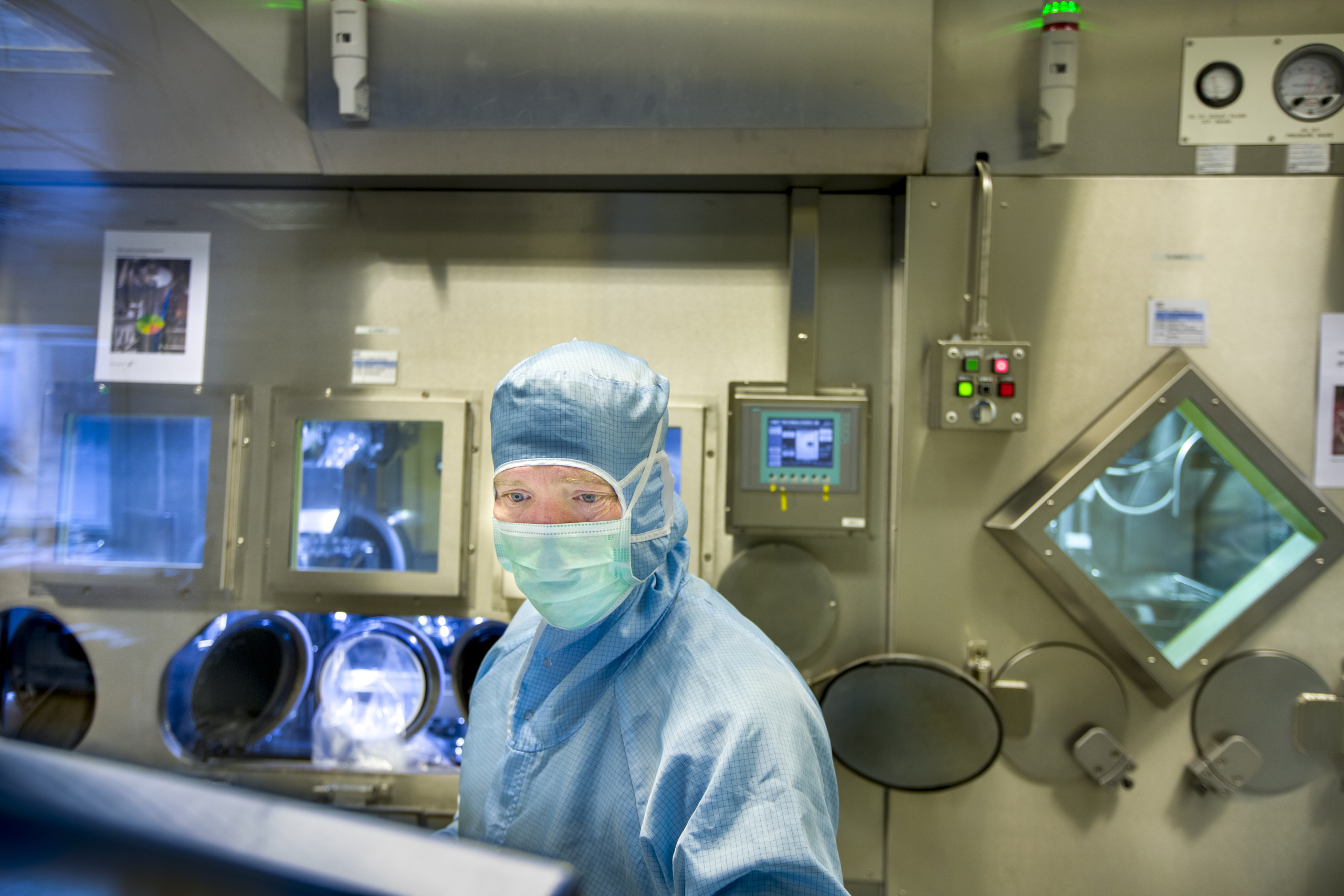
Agilera hasboth broad experience and cutting-edge expertise in the development, production and distribution of radiopharmaceuticals. Our core values – AGILITY – SAFETY – INNOVATION – PARTNERSHIP – are our hallmark, and they will help us, together with our customers , to realise patients’ access to the cancer medicine of tomorrow.
At Agilera, we strive to ensure a good, competence-oriented working environment, with plenty of opportunities for all employees to take part in exciting developments. As a new company we are building on the strong position we have developed as part of the Institute for Energy Technology (IFE). We have high ambitions for further development, which will enable us to be part of a market that is expanding and experiencing strong growth.
Our employees and areas of expertise
Our employees represent numerous areas of expertise and a wide range of experiences and backgrounds. We are involved in exciting specialist fields, such as radiopharmacy, radiochemistry, analytical chemistry, biochemistry, radiobiology, molecular biology, microbiology and radiation protection, and many others.
We have experts working with production processes that are subject to particular requirements for handling safety-critical procedures. We also have dedicated personnel involved in wholesale and retail processes, such as procurement, import, packing, warehousing and export, where we have particularly stringent requirements in terms of timing and safety. Our quality assurance team is highly skilled, and includes Qualified Persons (QPs) and coordinators who ensure compliance with regulations for pharmaceuticals in the EU; GMP (Good Manufacturing Practice) and GDP (Good Distribution Practice).
We are project managers, analysts, researchers, engineers, technicians, advisors and technical directors with academic degrees at all levels. Together, we make up the team that produces and distributes critically important radiopharmaceuticals for the world market and is involved in the development of tomorrow’s solutions within our specialist areas.
Even if none of our current vacancies fit your profile, we are always interested in new talent, and welcome open applications.